Radiation and Radioactive Decay
Radiation is the most common terminology that we often hear in our day-to-today life. The major source of natural radiation which we witness is from the Sun. The man-made radiation is from a nuclear reactor. There are various types of radiation and are classified based on their penetrating power. Some radiations are dangerous, and some are extremely useful. Radiation plays a major role in imaging science and curing deadly diseases like cancer using radiation therapy and many more. Radiation is used to kill cancer cells and helps to cure the disease.
There are three major types of radiation: Alpha, Beta, and Gamma. A material is said to be radioactive if it contains unstable nuclei. Radioactivity is the process by which an unstable atomic nucleus loses energy by radiation. There are three types of radioactive decay; they are alpha decay, beta decay, and gamma decay. In this session, let us learn about alpha decay and beta decay.
Alpha Decay
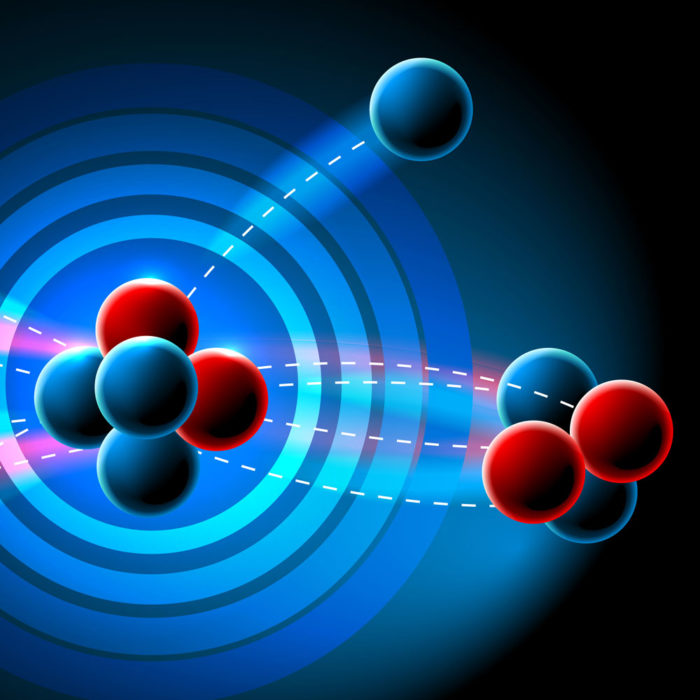
Alpha decay (α-decay) is the most common nuclear decay. In this process, the nucleus emits an alpha particle, or a particle containing two protons and two neutrons. Alpha decay was distinguished from other types of radiation by the famous scientist Ernest Rutherford. He studied the deflection of the radiation through a magnetic field, and the deflection of alpha decay would be a positive charge as the particles have a +2e charge.
Alpha decay is represented by the following equation:
Where,
X is the parent nucleus
A is the total number of nucleons
Z is the total number of protons
Y is the daughter nucleus
He is the released alpha particle
The best example of alpha decay is uranium 238 undergoes radioactive decay to form thorium 234 along with the emission of a helium nucleus.
Beta Decay
Beta decay (β-decay) is one of the types of radioactive decay where a proton of a radioactive sample is transformed into a neutron or vice versa. This happens inside the nucleus of the radioactive element. In beta decay, the beta ray is emitted from an atomic nucleus. In this process, a proton can be turned to a neutron or neutron to a proton. There are two types of beta decay: β+ decay and β- decay. The electron and the positron are generated to obey the law of conservation of charge. Beta decay occurs via weak interactions. Beta particles are mainly used in the medical field to cure bone and eye cancer.
In a beta plus radioactive decay reaction, the proton disintegrates to yield a neutron. It results in a decrease in the atomic number. Here, the nucleus gains a neutron and experiences a loss of a proton.
The equation of beta plus decay is
An example of beta minus decay is the decay of hydrogen-3 (tritium) into helium-3 with a half-life of about 12.3 years.
In the beta minus decay reaction, a neutron is transformed to yield a proton. This results in an increase in the atom’s atomic number.
The equation of beta minus decay is
An example of beta minus decay is the decay of carbon-14 into nitrogen-14, with a half-life of about 5,730 years.